You have always followed the laws and rules in sanitizing and disinfecting your businesses, adhering to the guidelines and input from health inspectors, but are you doing enough? Sanitizing and disinfecting have taken on even more importance and awareness recently. It’s important to remember Sanitizing efforts are only as good as their application, so testing the efficacy is critical in preventing the growth and spread of many contaminants.
Read the Full Article »
Shop Now
Purpose
This document offers general and specific information covering proper sanitization in restaurants, food processing facilities, and anywhere else food is commercially prepared or served. It includes comparison charts, how to choose and select the right sanitizer test kits and how to use them properly, along with information about the Food Code and highlighting requirement testing.
The goal of this document is to give Health Inspectors an easy reference for on this important food safety practice so that you can provide to food service workers with information and that can help them and their in-house employee training efforts. Our goal is to help you keep a safe environment for employees, customers, and families.
The Basics: Disinfection vs Sterilization
Disinfection:
Disinfection- eliminates nearly all recognized pathogenic microorganisms but not necessarily all microbial forms (e.g., bacterial spores) on inanimate objects.
Sterilization- is a procedure that kills all microorganisms, including high numbers of bacterial endospores. Sterilization can be accomplished by heat, ethylene oxide gas, hydrogen peroxide gas, plasma, ozone, and radiation (in industry).
Disinfection eliminates nearly all recognized pathogenic microorganisms but not necessarily all microbial forms (e.g., bacterial spores) on inanimate objects.
Disinfection is used to decontaminate items. The primary objective of decontamination is to reduce the level of microbial contamination so that infection transmission is eliminated; decontamination renders an area, device, item, or material safe to handle (i.e., safe in the context of being reasonably free from a risk of disease transmission).
Sterilization:
Sterilization is a procedure that kills all microorganisms, including high numbers of bacterial endospores. Sterilization can be accomplished by heat, ethylene oxide gas, hydrogen peroxide gas, plasma, ozone, and radiation (in industry).
Cleaning:
Cleaning is the removal of dirt, food, or other soil from a surface, usually accomplished by a detergent.
Sanitizing:
Sanitizing is the removal of pathogens to a safe level, defined as a 5-log reduction, or removal of 99.999% of organisms.
Sanitizing only works when it follows proper cleaning!
Temperature Sanitization
Temperature Sanitization in retail food facilities is accomplished by:
- Hot water manual operations by immersion at temperature of 171°F (77°C) for at least 30 seconds.
- Hot water mechanical operations by being cycled through ware washing equipment at a temperature of 180°F (82°C.)
- Conveyor machines require a 180F manifold temperature to yield a 160F plate temperature.
- Stationary machines require 165F manifold to yield a 160F plate temperature.
Chemical Sanitization
Chemical Sanitization: is accomplished by:
Chemical Sanitization
Manual or Mechanical
- Chlorine
- Quaternary Ammonium Compounds (Quats)
- Iodine
- Other
- Chemical manual or mechanical operations, including the application of sanitizing chemicals by immersion, manual swabbing, brushing, or pressure spraying methods.
- In order for sanitization to be effective, sanitizers need to be in contact with the equipment or surface for a sufficient amount of time, called the contact time, as specified on the sanitizer label.
- And finally, we have chlorine and quats, the most common food contact surface sanitizers, that we’ll discuss in more detail. Others that you may find include iodine and peracetic, or peroxyacetic, acid (PAA.)
Chlorine
Concentration Range
(ppm)
|
Minimum Temperature
pH 10 or less
|
Minimum Temperature
pH 8 or less
|
|
25-49
|
120F (49C)
|
120F (49C)
|
|
50-99
|
100F (38C)
|
75F (24C)
|
|
100
|
55F (13C)
|
55F (13C)
|
Chlorine compounds generally used in food surface sanitization are calcium and sodium hypochloride. The solution must have a minimum temperature based on the concentration and PH of the solution as listed in this chart.
Why chlorine is affected by pH
As pH increases, chlorine becomes less effective as a sanitizer. Chlorine itself is a strong base – has a high pH. If the pH of the solution is high, chlorine won’t “react.” It takes a lower pH, more of an acidic environment, to get the chlorine to react in the water and actually do what we want it to do; that is, sanitize.
While chlorine is effective for most bacteria, viruses, fungi, and bacterial spores, chlorine is not the best choice to breakdown organic matter and biofilms (groups of microbial cells held together by a kind of cellulose; basically, microbial slime). Those require scrubbing, acidic cleansers, and quats.
As pH increases, chlorine becomes less effective as a sanitizer.
- Chlorine itself is a strong base – has a high pH (7 – 14)
- If the pH of the solution is high or basic, chlorine won’t “react.”
- It takes a lower pH, more of an acidic environment, to get the chlorine to react in the water and actually do what we want it to do; that is, sanitize.
Chlorine Effectiveness
- Chlorine IS effective for most bacteria, viruses, fungi, and bacterial spores
- Chlorine IS NOT the best choice to breakdown organic matter and biofilms (groups of microbial cells held together by a kind of cellulose; basically, microbial slime). These require scrubbing, acidic cleansers, and quats.
Quaternary Ammonium Compounds (Quats)
Quats are surfactants as well as sanitizers. A surfactant is the reason that detergents work. It is a chemical compound that lowers the surface tension of water, making it easier for water to “stick” to a substance. That, in turn, makes the surface itself more readily available for cleaning. 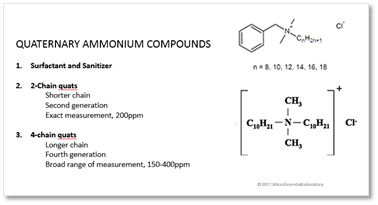
The 2 types of quats used in food safety sanitization are 2-chain and 4-chain quats. The number represents the number of “chains” coming off of the central nitrogen atom as shown in the following diagrams and can be written in many forms.
A quaternary ammonium compound solution must:
- Be tested at a temperature of 65°F - 75°F (18°C - 24°C) for greatest accuracy (this may not be the same as the “use” temperature.)
- Have a concentration as specified under the FDA Food Code § 7-204.11 and as indicated by the manufacturer’s use directions included in the labeling
- Be used only in water with 500 MG/L hardness or less or in water having a hardness no greater than specified by the EPA-registered label use instructions because the surfactant makes the quat more sensitive to water hardness levels.
- The way that the surfactant works, makes the quat sensitive to water hardness levels.
If a facility is on a municipal water system, the water supplier can provide you the hardness level of the water they deliver. If there is a private water supply, the water can be tested for hardness. Water that is above the 500 MD/L hardness-level may be treated with a water-softening system to reach appropriate levels
If a cloth is soaking in solution, there may not be enough sanitizer for it to be effective in reducing the number of pathogens.
- Quats bind to fabrics (microfiber cloths bind less than cotton)
- Sanitizer is “in the fabric” no longer in the solution
- Leads to an ineffective level of sanitization on the surface/equipment
- The Quat solution need to be tested just prior to use
Quat Binding
The sanitizing component in the quat is attracted to and absorbed into fabrics, making it inert. This happens when a cleaning cloth is left soaking in a quat solution.
The results are that the quat meant to sanitize a surface does not actually end up on that surface, but “bound” in the cloth. One study found that the level of sanitization decreased by 50% after soaking for just 10 minutes.
Therefore, when using quats, it is important to spray and wipe, dip, and wipe, or soak an item to be sanitized in a solution that has been tested and confirmed to be at the correct concentration.

Solution Differences
Facilities have to determine which sanitizer Is best for them; of course, using only FDA approved, EPA registered food-safe sanitizer chemicals.
|
SOLUTION
|
PROS
|
CONS
|
|
Chlorine
|
Highly effective on a wide variety of bacteria, inexpensive, and not affected by water hardness
|
Corrosive and irritating to the skin, effectiveness depends on solution pH and exposure to light; loses effectiveness with biofilms/organic matter, requiring more frequent water changes
|
|
Quats
|
Nontoxic, odorless, colorless, noncorrosive, nonirritating, effective over a wide pH range and with organic matter, heat stable
|
Works slower than chlorine, temperature sensitive, ineffective with hard water and some detergents
|
If a chlorine-based sanitizer is preferred, hypochlorites, either granular or in tablet form, are an option. Never use scented bleach.
Also, keep in mind that quaternary ammonium is different than ammonia.
Test Kits
If the effectiveness of chemical sanitizers is determined primarily by the concentration and pH of the sanitizer solution, and those values can vary, a test kit is necessary to accurately determine the concentration of the chemical sanitizer solution.
And, there are different sanitizing test kits for the various chemical sanitizers.
- QT-10, or “Quat Test – 10” is formulated for 2-chain quat compounds with measurements in the range of 0 - 400ppm.
- QT-40 is a quat test paper for 4-chain quats with measurements of 0 - 500ppm.
- QAC is a general, non-specific match to test for all quats.
- Chlorine test papers provide a means to measure the concentration of free available chlorine in solution. Measurements range from 10 - 200ppm.
QAC test strips are not calibrated precisely to specific quat chemistries as the first two are.
Sanitization Needs
The WHERE in food safety sanitization can be split in to two parts:
- Where – in which types of facility – is sanitization needed? AND
- Where – within a facility – would there be sanitizer solution to test?
Facilities That Require Food Sanitation
Sanitizers are needed in all kinds of facilities, including but not limited to:
A small mom & pop restaurant might opt for household bleach because it’s cheaper, easier to access, and familiar, while fast food restaurants are more likely to use granulated chlorine. Larger restaurants and fast-food chains likely use quats for sanitizing.
- Restaurants
- Convenience Stores
- Schools
- Veterinary Clinics
- Tattoo Parlors
- Hair Salons
- Food Processing
- Prison Cafeterias
- Hospitals
Anywhere food is commercially prepared
Different facilities will use different types of sanitizer, depending on access, quantity, and cost.
Facility Areas that Need to be Sanitized and Tested
 Test chemical sanitizers in all locations. This includes, but not limited to:
Test chemical sanitizers in all locations. This includes, but not limited to:- the buckets for wiping cloths,
- 3-compartment sinks,
- spray bottles,
- low-temperature dish machines.
When to Test
Every time a solution is made or changed it needs to be tested. When should solutions be changed?
- Three-compartment sinks – Test every 2-4 hours or more frequently as usage requires (Soiling reduces effectiveness. See photo). Consider testing at the end of the washing process to ensure that the final item in the sanitizing rinse has been effectively sanitized.
- Buckets - Every 2 to 4 hours or more as needed to keep the water clean and the sanitizer effective in use.
- Spray Bottles-1 time/shift at minimum
- Dish machines- 1 time/shift at minimum
- Pre-soaked disposable sanitizer wipes- once per shift.

Inspecting the Tests and Testers
First ask to see the test strips and have a manager or employee test the sanitizing solution. Ensure that the employee starts with the right sanitizer and follows directions. Asking them to provide the strips will show you if they keep them readily available… an employee scrambling to find them is a bad sign!
Then watch them do the test. This will show you if they know how.

Ensure the facility has the correct kit for their sanitizer solution(s). Test kits are not interchangeable. Also, kits can expire - so be sure to check the date on the dispenser as well.
Ask to See their Test Kits

Ensure the facility has the correct kit for their sanitizer solution(s). Test kits are not interchangeable. Also, kits can expire - so be sure to check the date on the dispenser as well.
Examples of “Bad” Test Kits

This is an example of an expired kit. Expired kits should be thrown away.

Kits without dates were made long ago, before expiration dates were printed. They expired years ago.

Here is a new kit - still wrapped in foil. If this is presented as the active kit, they likely aren’t testing. At 180 inches, there is little likelihood that they are actually just starting a new roll.

These both show damaged or contaminated kit because it shows oxidation caused by exposure to air, humidity, or chemical fumes.
The first kit has likely been exposed to the air, humidity, or chlorine gas for too long. If you see a test paper roll that resembles the roll in front, you can be safe in assuming it is NOT being used in the facility.
You can tear-off about 2 inches after the end of the oxidation damage and test if it's still possible to use. Otherwise it's best to just dispose of the roll.
How to Test
Depending on the sanitizer used, you’ll need to pay attention to:
- Water temperature
- Foam and swishing
- Time
- Blotting
- Color chart
- Reading the results
We’ll review testing Quats and Chlorine
Quats
- Review the directions:

- Does the sanitizer require a specific water temperature?
- The general recommendation for quats is to be tested at room temperature, 65°F-75°F (18°C-24°C).
- Use a pre-cut test strip or tear off approximately 2 inches of the roll of test paper.
 Quats will often foam when filling a sink or bucket with solution. Avoid testing the foam by either testing a clear area or letting the foam dissipate prior to testing.
Quats will often foam when filling a sink or bucket with solution. Avoid testing the foam by either testing a clear area or letting the foam dissipate prior to testing.
- The foam has a higher concentration of sanitizer and therefore gives incorrect elevated results.
- NO swishing either! Again, it will force chemical into the strip and give a false high reading or give a modeled affect to variable uptake of the sanitizer.
 Make sure the foam is gone, and the test strip goes directly into the water. Dip the strip in to the sanitizer water.
Make sure the foam is gone, and the test strip goes directly into the water. Dip the strip in to the sanitizer water.- Leave the strip in the solution for the correct amount of time based on the type of sanitizer being used (usually 10 seconds). Do not swirl or move the strip – hold it still.
- Remove the test strip and immediately compare it to the color chart.
 The color chart is conveniently found on the test strip dispenser. To determine the concentration of sanitizer, match the color of the strip after dipping to the closest color on the chart provided. Each color represents a different sanitizer concentration in parts per million, or ppm.
The color chart is conveniently found on the test strip dispenser. To determine the concentration of sanitizer, match the color of the strip after dipping to the closest color on the chart provided. Each color represents a different sanitizer concentration in parts per million, or ppm.- If a quat reading is between 150ppm and 400ppm for 4-chain quats, then the concentration is fine.
- 2-chain quats generally 200ppm “not to exceed” 400ppm.
Chlorine
Testing chlorine solution is only slightly different.
- Review the directions.

- Does the sanitizer require a specific water temperature?
- As we saw in the table earlier, the minimum temperatures for chlorine solutions can vary based on the pH of the solution.
- Room temperature, 65°F-75°F (18°C-24°C), is still a safe bet, as are solutions with cooler temperatures. The warmer the water in a chlorine solution, the lower the concentration of available chlorine is needed to show a “sufficient” amount of sanitizer. So, if the water is not too hot, the test will work correctly.
 The table provided chlorine concentrations based on the solution having maximum pH values of 8 or 10. The water supply should ensure the pH never go above those levels, but pH meters are available for intermittent testing.
The table provided chlorine concentrations based on the solution having maximum pH values of 8 or 10. The water supply should ensure the pH never go above those levels, but pH meters are available for intermittent testing.
- As with the quats, use a pre-cut test strip, or tear off approximately 2 inches of the roll of test paper.
- A chlorine test strip can be dipped and promptly removed – without waiting the 10 seconds as needed for the quats.
 Make sure to read the directions - some chlorine test kits require you to “blot” the strip on a paper towel prior to comparing to the color chart.
Make sure to read the directions - some chlorine test kits require you to “blot” the strip on a paper towel prior to comparing to the color chart.
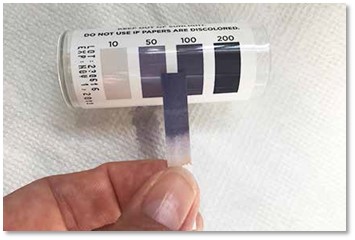 Chlorine bleach should be between 50 and 100ppm.
Chlorine bleach should be between 50 and 100ppm.- The chemical manufacturer generally determines the concentration for effective sanitization; read the label.
Testing Tips
 When testing either a quat or chlorine solution, if the concentration is too low or too high, add sanitizer or dilute with water as needed in order to achieve the required concentration.
When testing either a quat or chlorine solution, if the concentration is too low or too high, add sanitizer or dilute with water as needed in order to achieve the required concentration.
Be sure to retest.
Remember to test following the cleaning and sanitizing of wares also to determine if the solution is still at the proper sanitization level. You need to ensure that the last items sanitized were done at the correct concentration too - not just the first ones.